Products & Services
Sensors, Instruments, and Services
At Gasporox, we deliver more than just cutting-edge technology. We provide complete solutions for non-destructive gas measurement. Our portfolio includes high-performance laser-based sensors, dedicated instruments, and a full range of services to support your inspection needs across pharmaceuticals, food, and beverage packaging during production and development.
From inline sensor integration to standalone devices and GMP-compliant software, every product is backed by expert calibration, training, and service support. All of this is designed to ensure maximum quality, compliance, and confidence in your production line.
Instruments

GPX1500 Vial
A compact, easy-to-use tabletop instrument for fast, accurate, non-intrusive, and non-destructive headspace analysis (HSA) and container closure integrity testing (CCIT) of vials and ampoules.

GPX1500 Film Pharma
Ideal for at-line or laboratory use, offering fast, accurate, and non-intrusive oxygen gas inspection. This instrument is a compact, easy-to-use tabletop instrument for non-destructive headspace analysis (HSA) and container closure integrity testing (CCIT) of intravenous bags and pharmaceutical pouches.

GPX1500 Film Food
A non-destructive testing instrument for oxygen (O₂) and carbon dioxide (CO₂) analysis in flexible film food packaging using Modified Atmosphere Packaging (MAP). Ideal for at-line or research use, offering fast, accurate, and non-intrusive headspace analysis (HSA) and quality control.
Machines
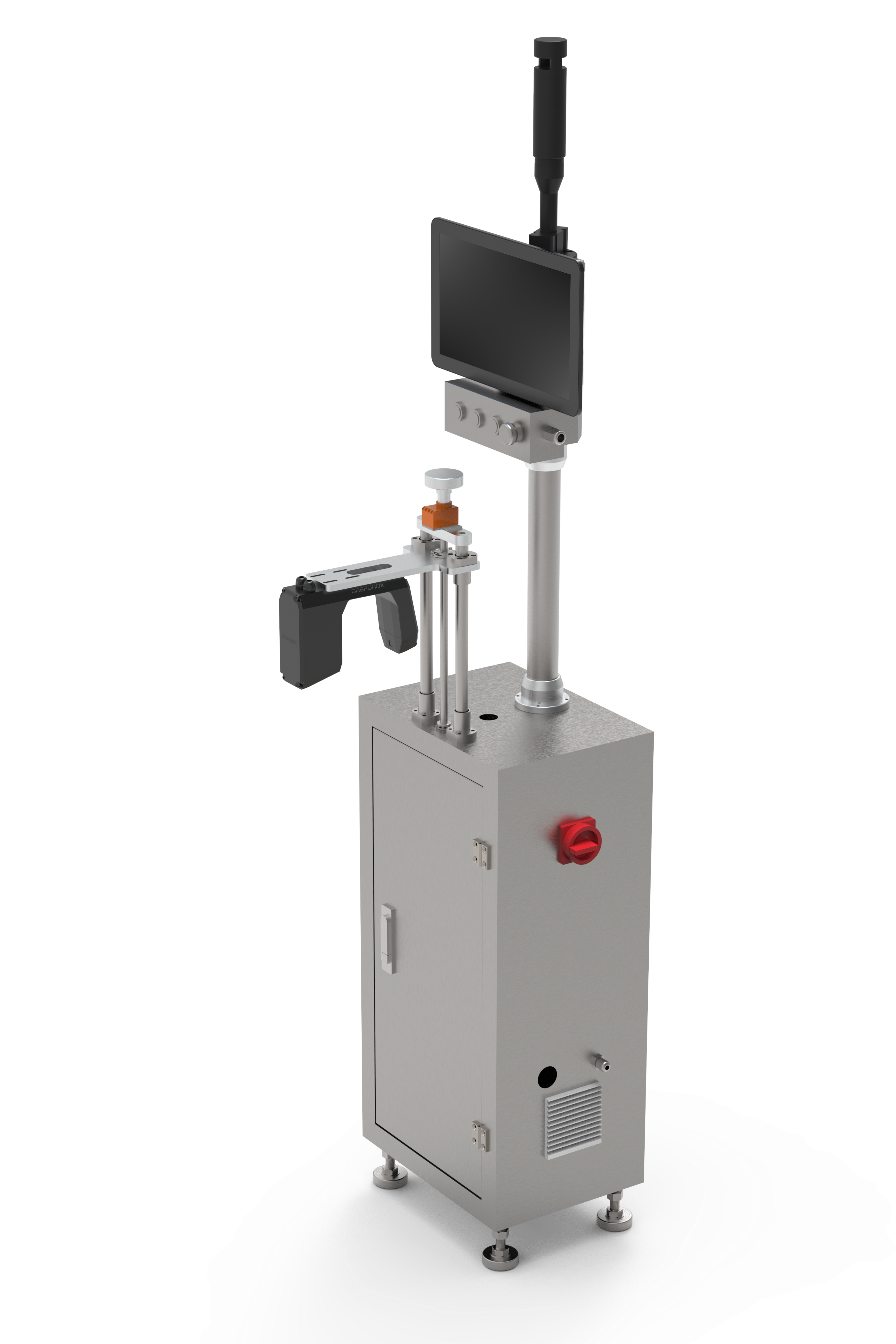
LineArch
A machine designed for easy integegration of headspace analysis (HSA) and container closure integrity testing (CCIT) in-line pharmaceutical manufacturers. Capable of measuring gases and pressure on a wide variety of containers such as ISO standard vials, bottles, and ampoules but also customized customer packaging.
Sensors

VialArch™
A ready-to-implement solution for in-line headspace analysis (HSA), container closure integrity testing (CCIT), and residual oxygen (O₂) measurement of vials from 2R to 100R. Designed for seamless integration into inspection machines and automated production lines.

BottleArch™
A ready-to-integrate sensor module for in-line headspace analysis (HSA) and container closure integrity testing (CCIT) of vials, ampoules, and large volume parenterals. Uses laser-based spectroscopy to enable non-destructive, 100% in-line residual oxygen (O₂) measurement and gas ingress detection, designed for GMP-compliant pharmaceutical production lines and OEM inspection systems.

AutoMAP™ Pharma
An integration-ready sensor module for pharmaceutical Modified Atmosphere Packaging (MAP), offering non-destructive, in-line headspace analysis (HSA) and container closure integrity testing (CCIT) for large and small volume parenterals (LVP/SVP). Utilizing TDLAS technology, it ensures precise oxygen (O₂) measurements from 0–100%.
AutoMAP™ Food
A sensor module ready for integration, designed for quality control and residual oxygen (O₂) measurement in food-grade Modified Atmosphere Packaging (MAP). It provides fast, accurate, and non-destructive in-line headspace analysis (HSA) of film-sealed food products.

CellSpect
An advanced non-destructive gas measurement system engineered for highly sensitive CO₂ detection in leak testing applications. Capable of identifying even the smallest microleaks, it ensures reliable quality control for packaging formats such as trays, bags, and cans – regardless of material type.
Laboratory services
Alpha Studies
Gasporox Alpha Studies offers fast, lab based feasibility testing to evaluate the compatibility of products and packaging with Gasporox headspace analysis and CCIT technology. The service supports product development, validation, and innovation through non destructive testing, documented results, and expert guidance, with pathways for both product evaluation and technology development across pharmaceutical, food, and beverage applications.
BatchCheck
BatchCheck is a non-destructive laboratory service for batch level verification of headspace gas composition and package pressure in sealed products. Manufacturers submit a full batch or representative samples for laser based analysis of oxygen, carbon dioxide, and total or partial pressure at a defined time point. The service delivers clear, traceable results to support quality assurance and batch release decisions without product loss or investment in in-house equipment, with intact samples returned for sale, storage, or further use.
Multi-parameter analysis
Multi-parameter analysis is a laboratory service for integrated, non-destructive evaluation of headspace gas composition and internal package pressure. By combining oxygen, carbon dioxide, and pressure measurements within a single, tailored study, the service provides a comprehensive view of packaging and product behaviour over defined time points and conditions. It supports development, optimisation, and investigation work by delivering quantitative, repeatable data that reveals interactions between gas composition, pressure, and packaging performance across pharmaceutical, food, and beverage applications.
Stability studies
Stability studies are long term, non-destructive laboratory evaluations of how packaged products and their packaging perform during storage. By repeatedly measuring headspace gas composition and internal package pressure at defined time points, the service provides trend based insight into shelf life, modified atmosphere performance, and long term package integrity. Stability studies support data driven decisions for packaging selection, shelf life definition, and performance verification across pharmaceutical, food, and beverage applications.
Customer Defined Studies
Customer Defined Studies are fully bespoke, non-destructive laboratory studies tailored to applications that fall outside standard service offerings. Each study is designed in close collaboration with the customer to address specific technical, development, or investigation needs, with customised measurement parameters, study scope, and reporting. The service delivers targeted, decision relevant data using laser based headspace analysis and package integrity measurements, without the need for capital equipment investment, and supports complex or exploratory work across pharmaceutical, food, beverage, and packaging applications.
Other products and services

Service & Acessories
Gasporox offers more than sensors – we provide complete support for pharmaceutical quality assurance, including IQ/OQ protocols, remote calibration services, training, and customised accessories for the GPX1500.
GPX HMI Software
Our GMP-compliant GPX HMI software ensures secure data management and full traceability (CFR21 Part 11), reducing integration time with user-friendly operation and robust electronic recordkeeping.
Technology
The Gasporox sensors and instruments are built on more than 25 years of development and refinement in industrial laser-based gas measurement. The system uses Tunable Diode Laser Absorption Spectroscopy (TDLAS), a highly reliable and precise method for non-destructive headspace gas analysis.
Developed by Gasporox in collaboration with its technology partners, this laser sensor technology is widely used in demanding industries such as pharmaceuticals, petrochemicals, and energy.
Gasporox’s laser-based headspace analysis has been successfully deployed for over a decade in pharmaceutical manufacturing. It is integrated into fully automated inspection systems and benchtop instruments for 100% in-line and laboratory testing of vials, ampoules, and other parenteral packaging formats. The performance and robustness of the system have been validated through extensive real-world use.

