LineArch
In-line machine for CCIT and HSA of pharmaceutical parenterals
Fast, Accurate, and Non-Destructive — Ready for in-line integration
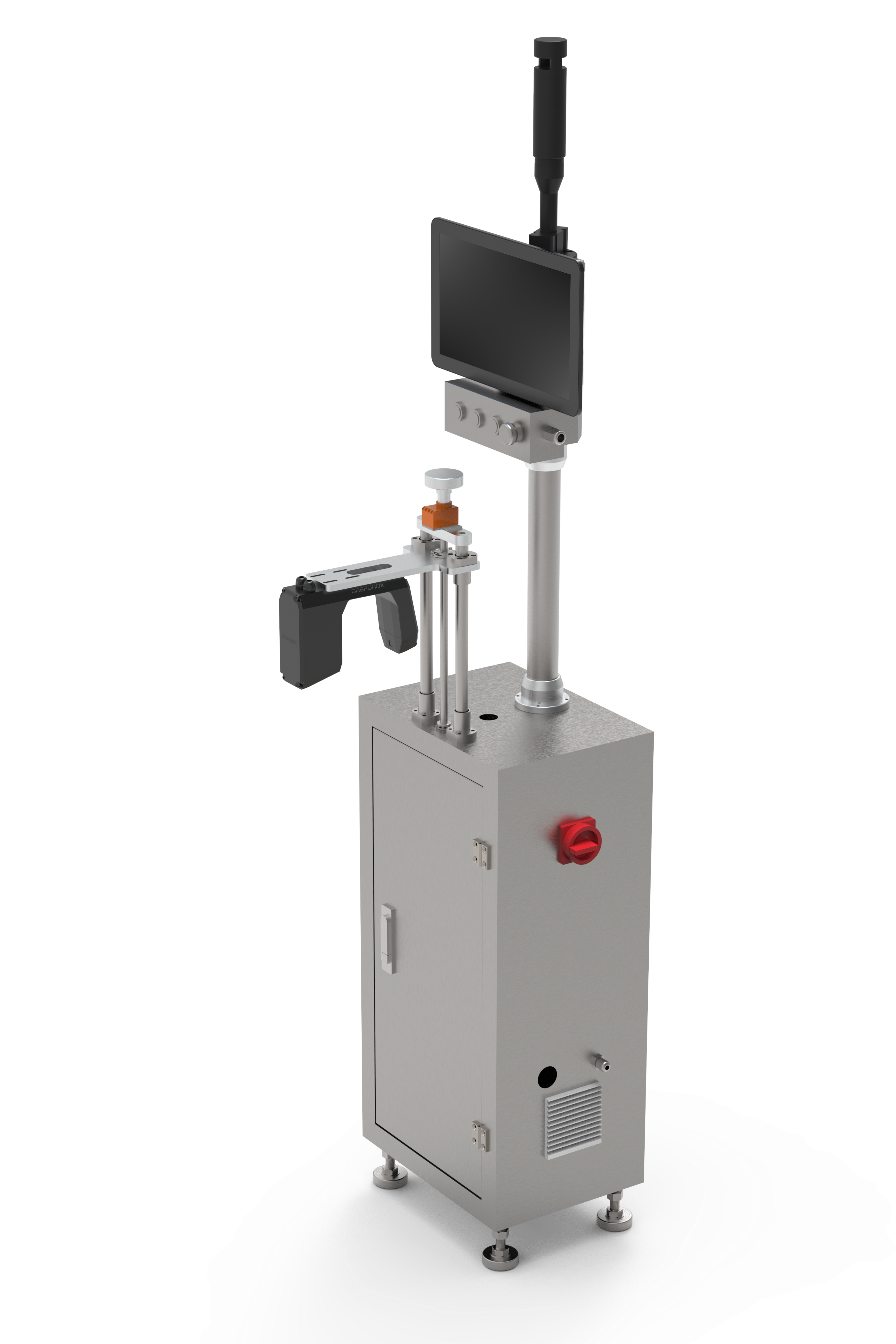
The LineArch is an integration-ready solution offering non-destructive headspace analysis (HSA) for direct installation into production lines, and thanks to its small footprint it can even be retrofitted into existing production lines allowing for easy yet impactful upgrades to pharmaceutical quality assurance.
Powered by proven advanced laser-based optical spectroscopy utilizing the VialArch™ and BottleArch™ sensors it enables up to 100% in-line quality inspection of residual gases (depending on variation) and supports the validation of container closure integrity testing (CCIT). This is essential for detecting gas ingress in oxygen sensitive and antiseptic pharmaceutical products.
How does it work?
As containers pass through the sensor module of the machine on the conveyor belt, a laser is shot through the headspace and effectively scans the headspace of each container, analyzing its gas content. This allows for fast, accurate, and non-destructive in-line container closure integrity testing (CCIT) through headspace analysis (HSA), without the need for consumables or purging.
Thanks to automatic triggering, setup is minimal. The only parameters required are container diameter and belt speed, which can be easily defined by the operator via the integrated user software or a connected control system.
Applications for the LineArch
The LineArch is designed for non-destructive inspection and quality assurance of pharmaceutical parenterals. Avaialble both with the VialArch™ or BottleArch™ sensors its capable of measuring a wide range of both large volume (LVP) and small volume parenterals (SVP). It is ideal for container closure integrity testing (CCIT) in the manufacturing of oxygen-sensitive drugs. Common use cases include measuring residual oxygen in liquid-filled containers and identifying leaks in lyophilized vials.
While optimized for pharmaceutical production, the LineArch can also be applied to other packaging formats requiring oxygen measurement and container integrity testing, provided compatability between sensor module and container.
Thanks to the easy setup time and small footprint combined with its versatility in being retrofitted into existing lines the LineArch can also function as a movable diognostic tool for use in maintance and troubleshooting of lines.
Contact Us
Want a quote or need more details about this product? Our team is ready to help.
Available Variants
The LineArch is available with any of the existing variations of the VialArch™ or BottleArch™ sensors mounted:

VialArch™
- VialArch™ O₂ – Measures residual oxygen and detects pressure changes caused by gas ingress.
- VialArch™ H₂O – Measures total and partial pressure levels.
- VialArch™ CO₂ – Developed upon request
Optional addons (available for all variations):
- Eject module

BottleArch™
- BottleArch™ O₂ – Measures residual oxygen and detects pressure changes caused by gas ingress.
- BottleArch™ H₂O – Developed upon request
- BottleArch™ CO₂ – Developed upon request
Seamless integration, Minimal effort
With its compact footprint on the production line the LineArch is an innovative and integration-ready solution designed for the pharmaceutical industry.It is easy to install, calibration free, and provides measurement sampling and evaluation directly within the module. This enables quick upgrades or retrofits to existing production lines while also providing a valuable addition to new production lines.
With the LineArch there is no need for daily maintenance or calibration due to its self-calibrating functionality. With minimal setup required, this solution is a plug-and-play allowing for the direct integration of headspace analysis (HSA) and container closure integrity testing (CCIT) into your production line, reducing recalls by increasing quality and consumer safety. Taking quality control a step beyond compliance.
Support and Optimization
All Gasporox products are delivered with the Gasporox Measurement Concept, offering complete integration support. Our team works closely with you to ensure the machine and is optimized for your specific product, container, and machine setup.
We are committed to helping you achieve the best possible performance across your entire inspection process.
- available downloads
-
Download technical data sheet
Key Features and Technical Highlights
- High-performance headspace analysis (HSA)/headspace gas analysis (HGA)
- Delivers accurate and reliable non-destructive measurements
- Compact design for seamless integration into production lines
- Compatible with a wide range of machine interfaces and automation systems
- Long operational lifetime with minimal maintenance
- Wide range of measurment capabilities and use cases
- Designed in compliance with Good Manufacturing Practice (GMP) guidelines
- Deterministic container closure integrity (CCI) test method according to USP <1207>
- Recomended test method according to Annex 1

Technology
The VialArch™ optical gas sensors are built on more than 25 years of development and refinement in industrial laser-based gas measurement. The system uses Tunable Diode Laser Absorption Spectroscopy (TDLAS), a highly reliable and precise method for non-destructive headspace gas analysis.
Developed by Gasporox in collaboration with its technology partners, this laser sensor technology is widely used in demanding industries such as pharmaceuticals, petrochemicals, and energy.
Gasporox’s laser-based headspace analysis has been successfully deployed for over a decade in pharmaceutical manufacturing. It is integrated into fully automated inspection systems and benchtop instruments for 100% in-line and laboratory testing of vials, ampoules, and other parenteral packaging formats. The performance and robustness of the system have been validated through extensive real-world use.
Explore More
GPX1500 Vial
Instrument for quality inspection and CCIT of pharmaceutical vials and ampoules.
GPX1500 Film Pharma
Non-destructive Headspace Gas Analyser for flexible pharmaceutical containers.
Vial Inspection
Lyophilized vial inspection with the GPX1500 Vial instrument.
